Boddingtons moves ahead with the Medical Device Single Audit Program (MDSAP) – learn more at MTI EXPO May 15-16!
Leading injection moulder and med-tech supplier, Boddingtons, has recently committed significant investment in order to transition to the Medical Device Single Audit Program (MDSAP). It is confirmed that this commitment will continue beyond the three-year certification cycle.
Boddingtons Quality Manager Jay Cheema says that ‘we are currently busy working on our Quality Management Systems and Regulatory Affairs processes in preparation for our MDSAP assessments scheduled to take place in May and July 2019.’
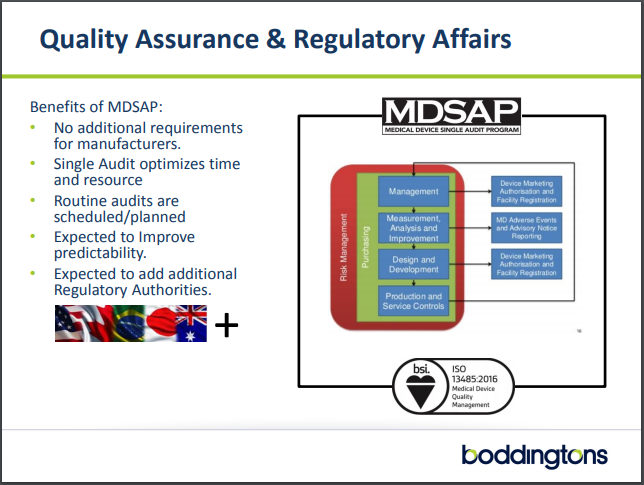
MDSAP is a global regulatory audit approach promoting alignment of Quality Management Systems and Regulatory approaches with the goal of improving medical device safety.
An overview of the MDSAP system
The Regulatory Authorities in participation include the Therapeutic Goods Administration (TGA) of Australia, Brazil’s Agência Nacional de Vigilância Sanitária (ANVISA), Health Canada, Japan’s Ministry of Health and Labour and Welfare (MHLW), and the U.S. Food and Drug Administration (FDA).
Boddingtons CEO Andy Tibbs will include MDSAP material in his May 15th presentation at MTI EXPO on the afternoon of the first day of the show at Birmingham’s NEC, May 15th.
Tibbs says that ‘we welcome the MDSAP approach and see additional benefit in structuring our systems to comply with ISO13485:2016, MDR and the quality and regulatory requirements of five participating authorities. We understand that additional authorities will sign up to this program and Boddingtons expects to see additional benefit when this happens.’
Boddingtons returns to the MTI EXPO, May 15-16 for the fourth year running with its biggest and best exhibition offering yet.
The 8m x 4m stand will play host to Boddingtons’s leading edge in medical manufacturing services.
Since the launch of its £4.6m manufacturing facility in early 2017, Boddingtons has steadily expanded the scope and volume of its med-tech manufacturing services. Rewards, qualifications and awards have followed. The company is certified to ISO13485 and ISO9001 Quality Management Systems Requirements and is also compliant with FDA 21 CFR Part 820.
Expert Boddingtons personnel will be at hand throughout the MTI exhibition to give detail on the full scope of the company’s manufacturing services, including MDSAP matters.